InfoScribe
From Clinical Requirements to Specifications
The Standards Selection Framework on InfoScribe provides users with the means to plan, choose and document interoperability solutions from concept through to implementation. Starting from the clinical requirements identified by clinicians through to business requirements, standards and technical specifications, the framework provides a comprehensive guide through the development of interoperability solutions.

Clinical Requirements describe the information and workflow needs of the clinician for a specific clinical context and clinical data exchange. Using the clinical Interoperability Principles as a guide, a set of requirements expressed in the clinician's voice will provide the foundation for a well-designed interoperability solution.

Business Requirements are derived from clinical requirements and provide a full picture of the solution that needs to be developed. Use cases, business rules and guidance are used to fully outline the solution design.
Standards Selection refers to the process that has been developed to help guide teams through the selection of terminology and messaging standards. Using the line of inquiry and considerations in the forms provided, implementation teams may assess the standards available and determine the best option for the point in time. The process also provides an opportunity for the InfoCentral community to share successful implementation projects, promoting standardization through reuse.
Standards are an integral piece of the interoperability solution, covering both the terminology that defines the data sent, and the messaging structures that define how the data is transferred. The framework provides the access and consideration criteria to the international and Canadian standards to facilitate implementation.
Why InfoScribe?
Online and Centralized
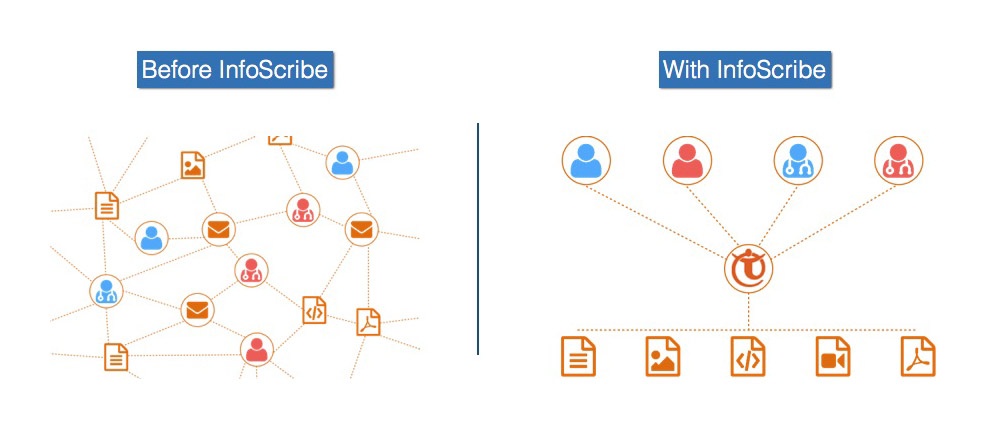
InfoScribe can be accessed from anywhere and at anytime with an internet browser. Also, content is stored online in one centralized location, which can be easily shared with team members and others. There’s no application switching or shuffling through emails to get the whole story – everything is in one place.
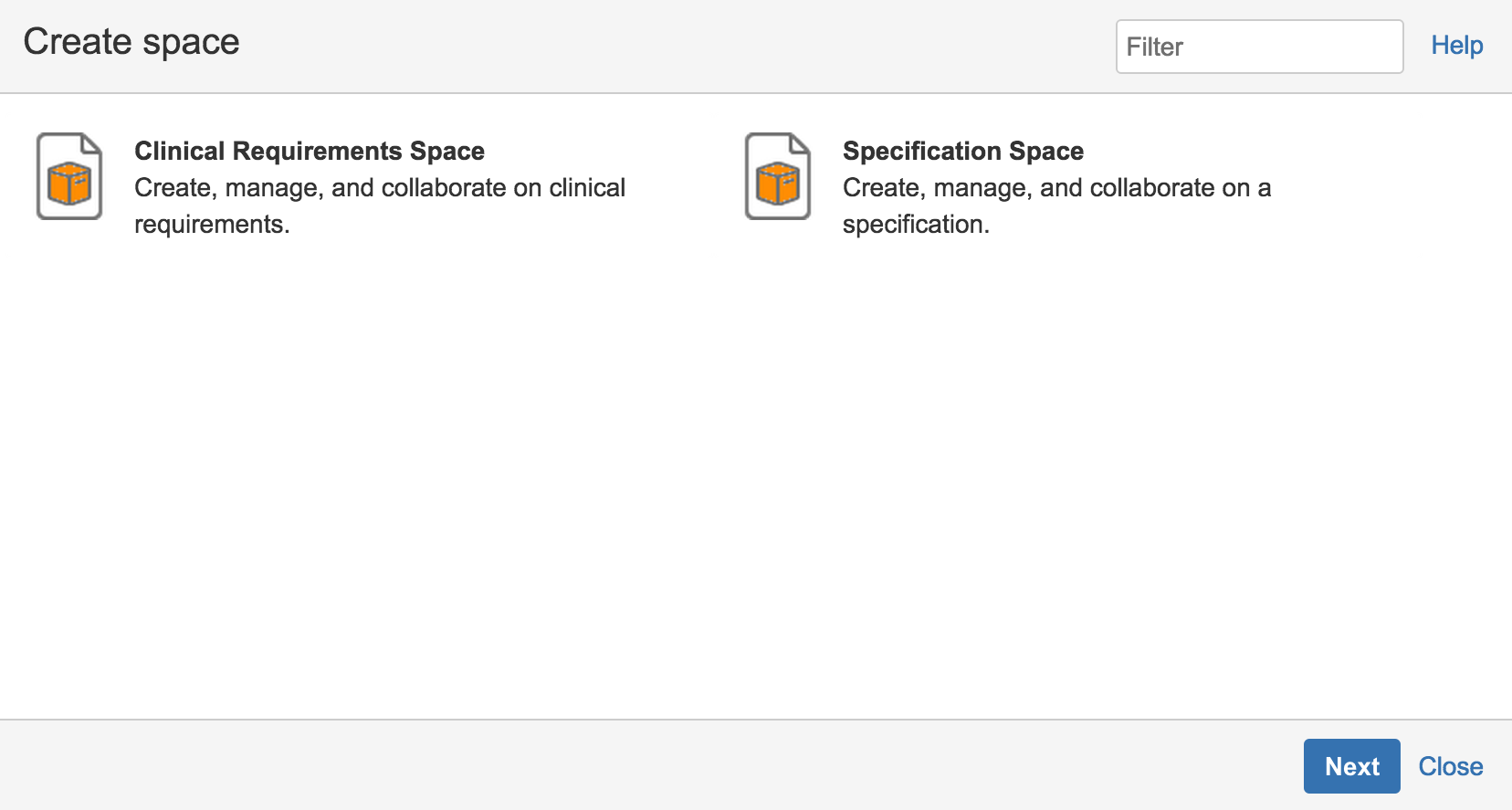
Get Started Quickly With Templates
Templates are integrated in InfoScribe to accelerate the development of clinical requirements and specifications. It ensures content is structured in a logical and consistent manner so readers can easily and quickly utilize the content.
Pinpoint Comments
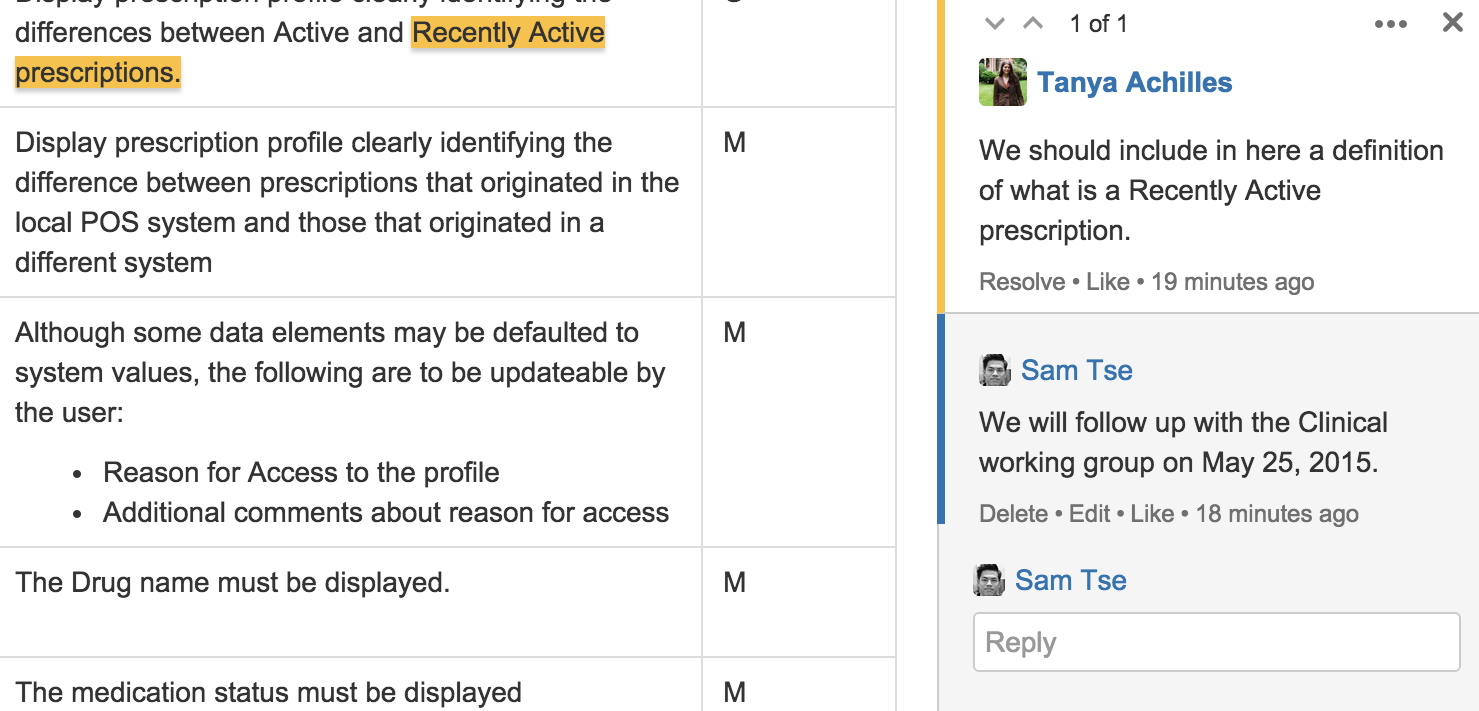
You can now give and discuss specific feedback anywhere on a page with inline comments:
- Highlight any amount of text – a letter, word, or paragraph – on a page to trigger an inline comment where you can give rich feedback,
- use @mentions to bring others into the discussion,
- add links to offer more context,
- or use emoticons to add some flavour.
Reviewing and collaborating clinical requirements and specifications is better than ever in InfoScribe.
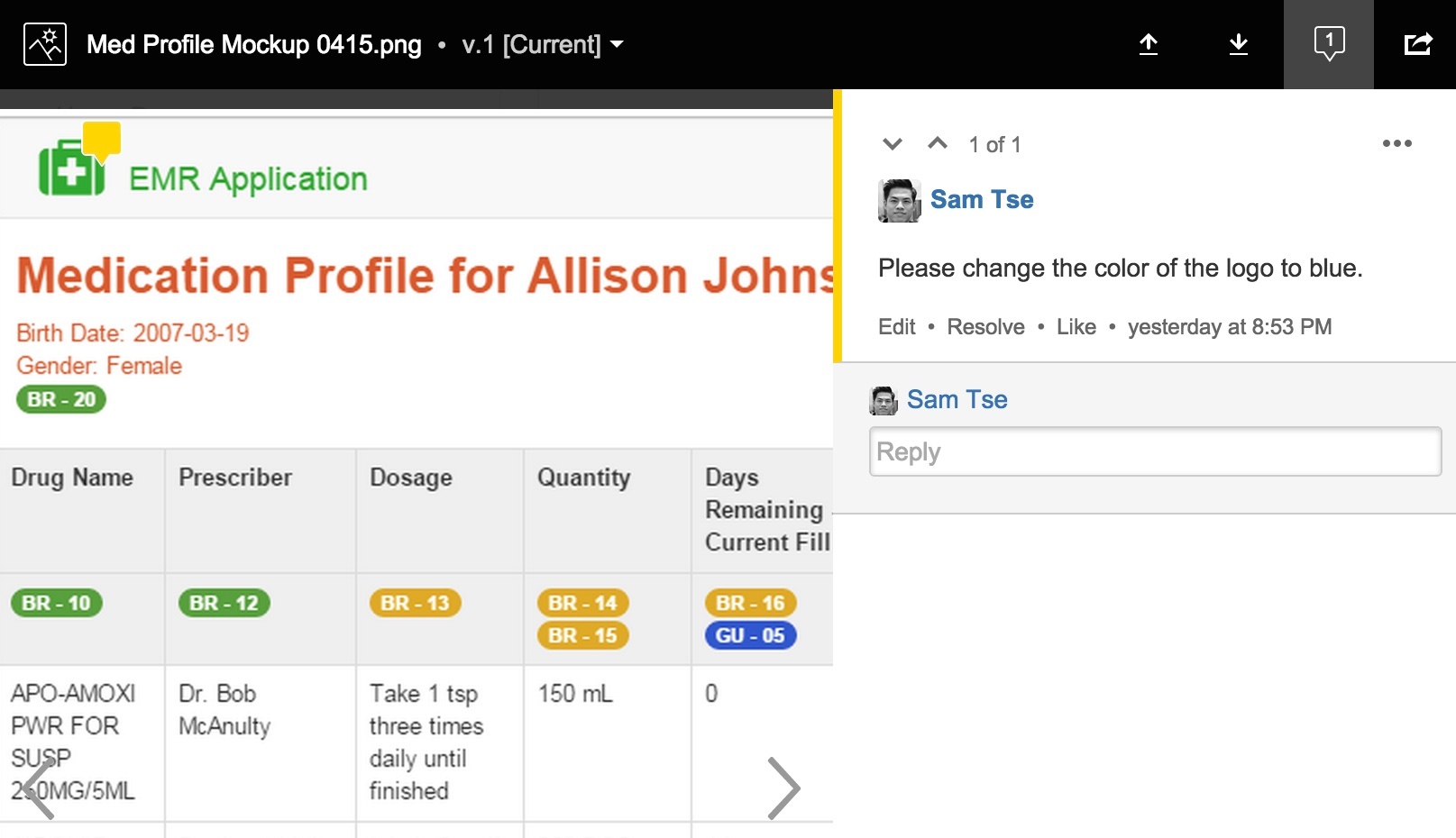
Furthermore, you can use the same comment functionality on an image as well, allowing you to pinpoint anywhere on the an image with inline comments.
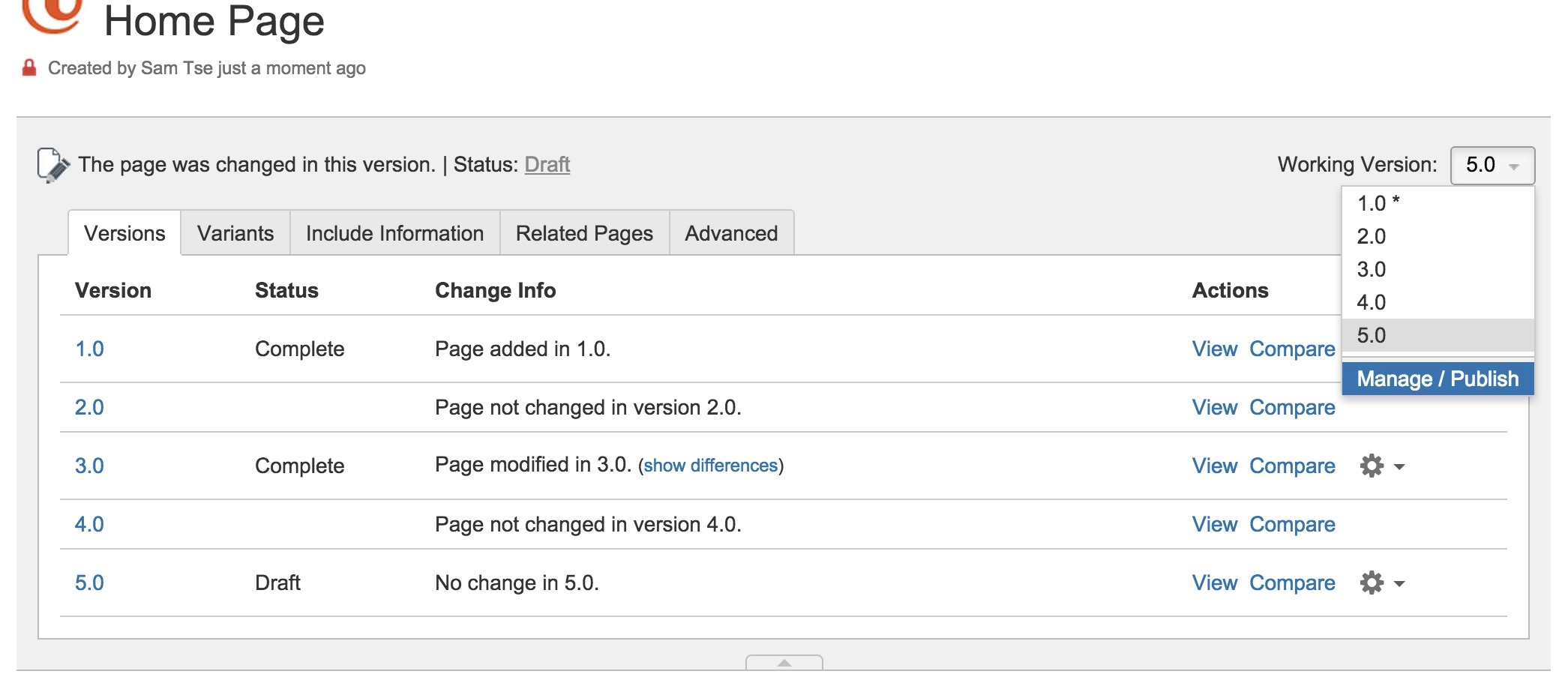
Versioning
InfoScribe allows you to manage and author multiple versions of your documentation in a single space. Select the specific version via a dropdown menu and immediately begin editing your content. When you're done, in just a few clicks to publish and share your content.
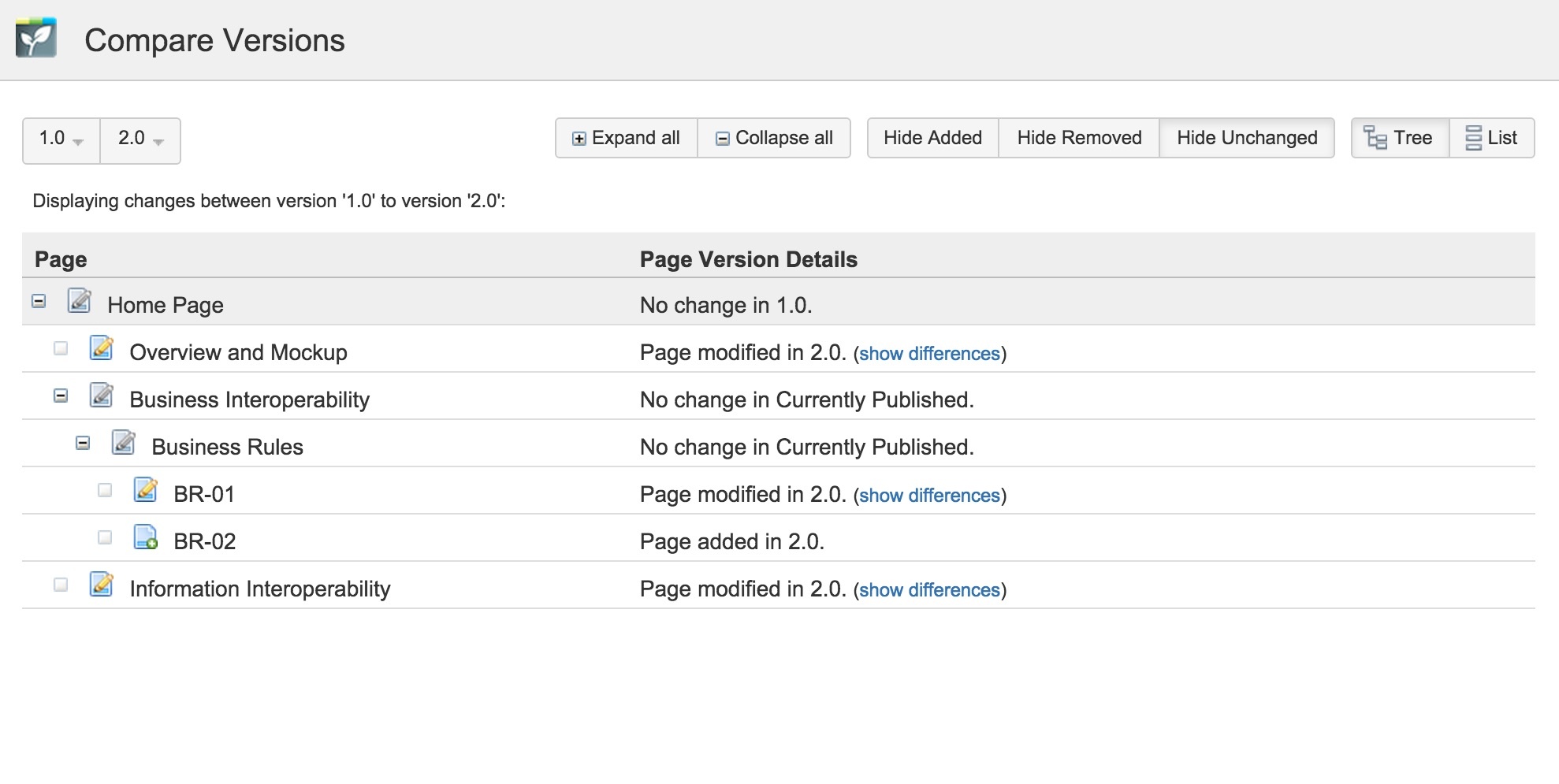
Furthermore, you can compare versions and find out the differences.
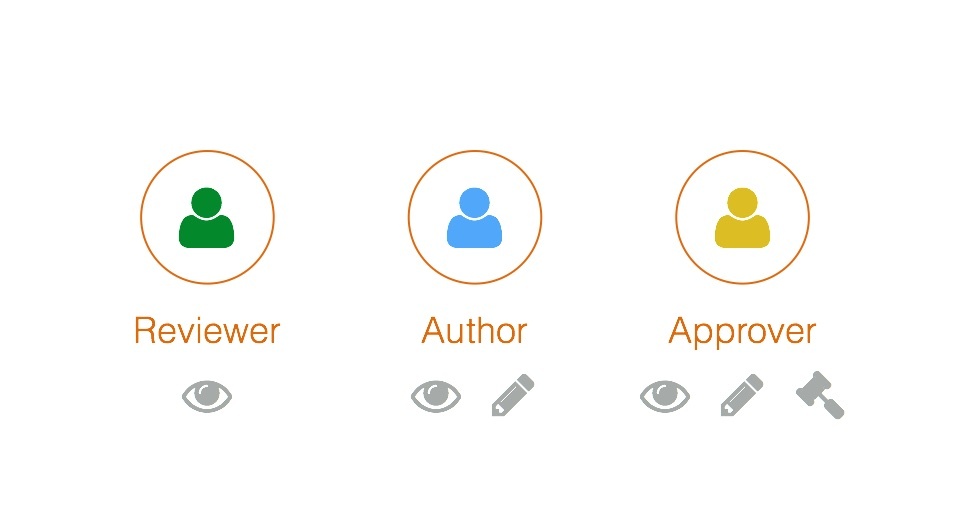
Roles and Permissions
Each user working on a space can be assigned to one of the following roles - reviewer, author, or approver - each has its own permissions and responsibilities. For example, reviewer can view all existing versions of the documentation. Author can contribute content. Approver can contribute, approve, and publish content.
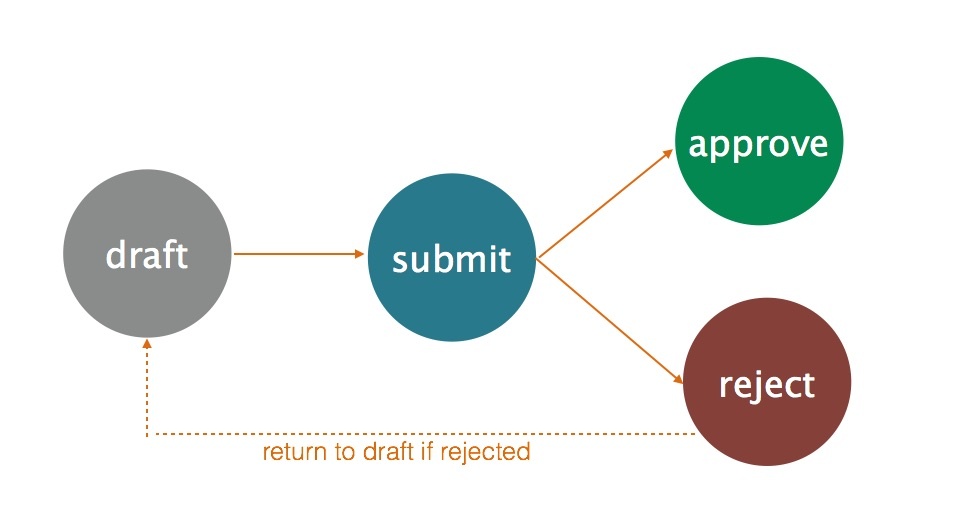
Simple Workflow for Publishing
Each page in a working space can go through the following states - draft, submit, approve, or reject. These states allow your team to manage the content's lifecycle at the page level and also to control what gets published during publishing. For example, InfoScribe provides the option to publish content that has been approved only. This option is useful if you have new content that should be excluded during publishing and you can do so by setting them to the draft or submit state. Also, the workflow is tied to the user's role. For example, an author can submit content for approval and an approver can approve (or reject) content.
Stay Up-to-Date with Email Notification
Always stay up-to-date with email notification when others made changes to content that you care about. You can control specifically what content you would like to watch and get notified via InfoScribe.
Export to PDF
InfoScribe supports PDF export, which consolidates all the pages in a space and properly formats all the content into a PDF document. It also supports hyperlinks which enable easy navigation from one spot to another in the PDF document. It is only a few clicks to export to PDF, it is that easy!