In support of the provinces and territories, Canada Health Infoway is facilitating a national collaborative effort to advance interoperability. While there are many interoperability-related challenges, this specification addresses secure sharing of Patient Summaries (e.g., Patient Summary-CA project)
Canada is not alone in trying to solve for this challenge. The International Patient Summary (IPS) project started in Europe several years ago and has been adopted by ISO, IHE and HL7 International. In addition, there is an active working group led by the Office of the National Coordinator (ONC) in the United States called the Global Digital Health Partnership (GDHP) that is actively working with member countries to find solutions for scaling Patient Summary exchanges at an international scale. Canada is an active participant in this partnership and has made a commitment at the G7 meeting in June 2021 to collaboratively work with jurisdictions, vendors, and participating organizations on a pan-Canadian effort to develop an implementable set of specifications aligned to the IPS that reflect Canada's jurisdictional realities. The overarching principle adopted for the Patient Summary-CA (PS-CA) project is to maintain as close of an alignment to the IPS profiles as possible while creating the instruments to allow jurisdictions to properly represent their desired clinical workflows and allow vendor systems to undergo necessary change management associated with adoption activities will make it a worthwhile investment.
The pan-Canadian Patient Summary-CA specification implementation approach for alignment with the IPS will span a number of releases on a roadmap. Release 1 will focus on three use cases, that have been identified as priority for Canadian jurisdictions (e.g., Alberta (AB), British Columbia (BC), Ontario (ON), Saskatchewan (SK) and Newfoundland & Labrador (NL)) and their supporting business requirements, actors and transactions, terminology and FHIR® profiles. This release will include supports for sharing Patient Summaries for scheduled or unscheduled local care with information from a single source.
Future releases will incorporate additional use cases and their supporting requirements, reference architecture, terminology and FHIR profiles. For example, the implementation roadmap will include a use case for creating the Patient Summary with information from more than one source and additional scenarios for supporting scheduled or unscheduled cross-border care.
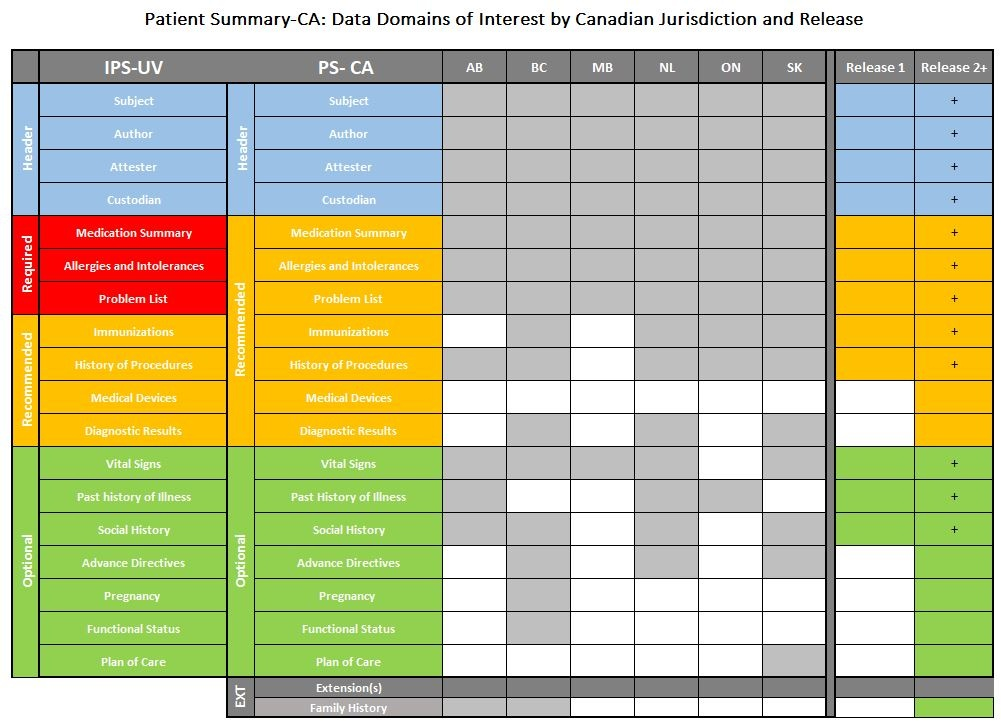
The following table represents the alignment of the PS-CA to the IPS-UV, data domains of interest by Canadian jurisdiction and the PS-CA Release 1 and 2 plans. Release 1 will include all of the data domains highlighted in the Release 1 column and Release 2+ will continue to build on the Release 1 data domains as well as add the additional data domains.
Legend
- Header domains are listed as blue
- Required domains are listed as red
- Recommended domains are listed as orange
- Optional domains are listed as green
Context
The pan-Canadian Patient Summary Interoperability Specification v1 Trial Implementation document is published to a public space within Canada Health Infoway’s InfoScribe and is also available as a downloadable document. InfoScribe is a web-based tool developed for jurisdictions and vendors to create, publish, and collaborate on clinical requirements and specifications for interoperability solutions. Teams can document, share, and discuss contents, files, ideas, specs, mock-ups, diagrams, and projects. A link to the online published content and the downloadable documentation will be published with each release of the Patient Summary-CA project.
Release information for each release is contained in the corresponding PS-CA Release page.
New content will be added throughout the life of the PS-CA Roadmap to accommodate the requirements of Canada's implementers.
Introduction to IHE
Integrating the Healthcare Enterprise (IHE) is an international initiative to promote the use of standards to achieve interoperability among health information technology (HIT) systems and effective use of electronic health records (EHRs). IHE provides a forum for care providers, HIT experts and other stakeholders in several clinical and operational domains to reach consensus on standards-based solutions to critical interoperability issues.
The primary output of IHE is system implementation guides, called IHE profiles. IHE publishes each profile through a well-defined process of public review and Trial Implementation and gathers profiles that have reached Final Text status into an IHE Technical Framework. These profiles are referenced in the Appendices of this document.
Preference for Modern HL7 FHIR interfaces
New implementation of IHE profiles based on the Patient Summary-CA standard should avoid legacy interfaces. IHE profiles that are HL7 FHIR based are preferred when available; however, the Reference Architecture will account for legacy systems that do not abide by FHIR. Canada Health Infoway will encourage the adoption of modern exchange protocols but will also provide the runway and opportunity for the jurisdictions to improve their interoperability capabilities.
How to Read This Document
This document contains the following content, as well as informative appendices for your convenience.
- Preface: Contains an introduction to the Pan Canadian Patient Summary Interoperability Specification v1 Trial implementation. This section contains a summary of the context, document purpose and scope, as well as other content to help orient the first-time reader to the topic of the Interoperability Specification and how it relates to other specifications in the digital health ecosystem in Canada
- PS-CA Use Case Overview: Describes the Use Case, including design constraints and assumptions and the flows of information that will be specified in this Interoperability Specification. Section 2 also introduces scenarios that describe how the specified flows may be used in the Canadian context.
- Core Interoperability Specification: Establishes the Core Interoperability Requirements for the Pan Canadian Patient Summary Interoperability Specification v1 Trial Implementation
- PS-CA Actor Conformance: Establishes the Conformance Requirements for the Interoperability Specification.
- Data Protection, Privacy & Security: Provides key considerations around Data Protection, Privacy and Security for the Patient Summary-CA specification.
- Information Models, Applications and Infrastructure: Provides key implementation guidance around Information Models, Applications and Infrastructure for the Patient Summary-CA specification.
- PS-CA Content Data Model & FHIR® Profiles: Describes the PS-CA Content Data Model & FHIR® Profiles required for the Pan Canadian Patient Summary Interoperability Specification v1 Trial Implementation
- Appendices: Contains supplementary information related to the IHE profiles and other related information for the Pan Canadian Patient Summary Interoperability Specification v1 Trial implementation
Related Documents & References
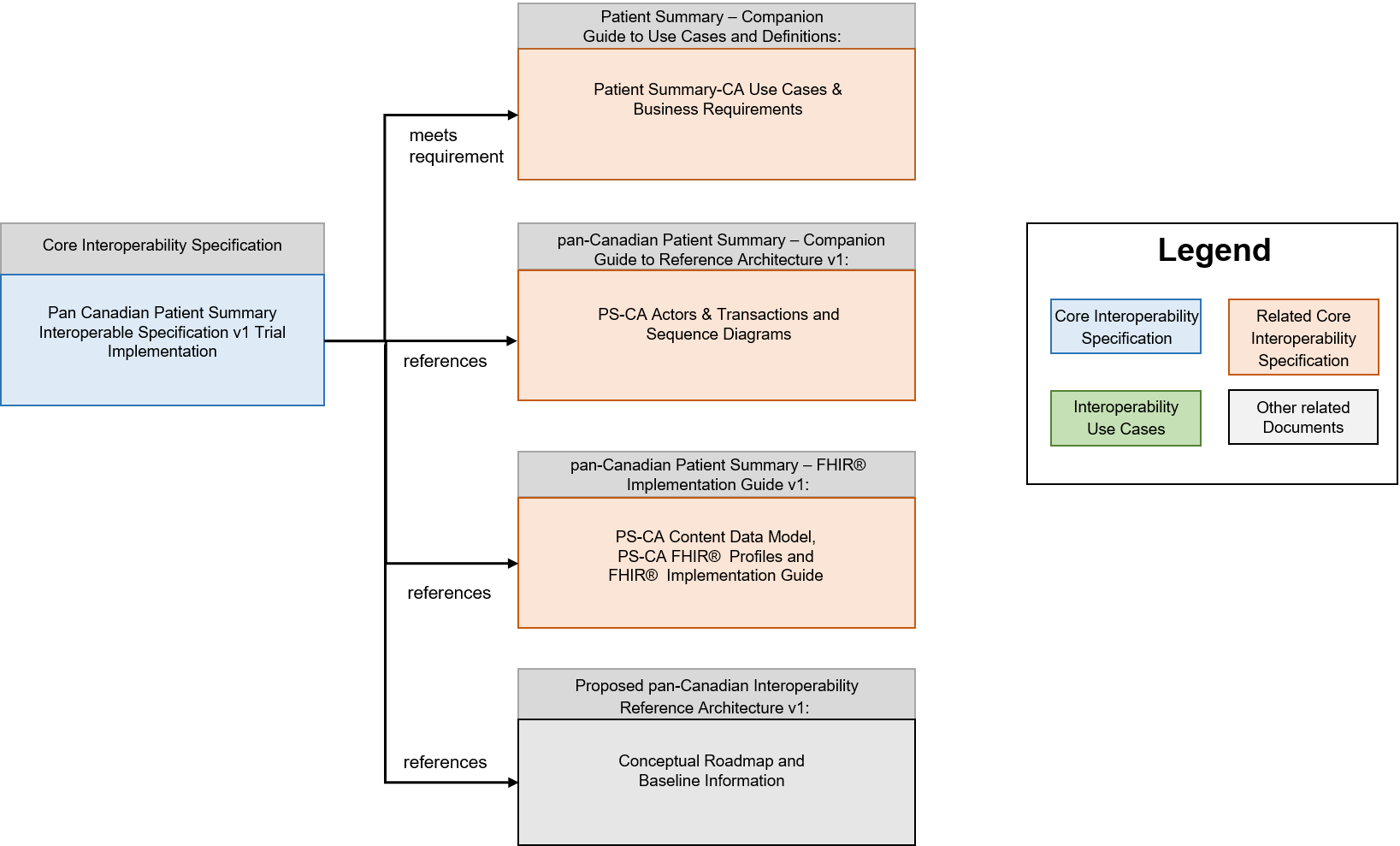
The Pan Canadian Patient Summary Interoperability Specification v1 Trial Implementation is the sole entry point for the technology developers, the compliance assessment testing and certification, and the purchaser of IT systems in terms of technical requirements
From this Interoperability Specification, several supporting documents are referenced:
- pan-Canadian Patient Summary – FHIR Implementation Guide
The pan-Canadian Patient Summary - FHIR Implementation Guide is an implementable, testable specification for the HL7 FHIR composition that defines the data payload of the PS-CA specification, based on the HL7 FHIR IPS implementation guide. It contains information for solution developers to implement the PS-CA content data model using the HL7® Fast Healthcare Interoperability Resources (FHIR®) standard. It describes the data elements & types, cardinality, constraints, and code system references - all of the details needed for two systems to be semantically interoperable with each other when a PS-CA compliant patient summary is exchanged.
Target Audience: Solution Developers
- pan-Canadian Patient Summary - Companion Guide to Use Cases & Definitions
The pan-Canadian Patient Summary - Companion Guide to Use Cases & Definitions, is a companion document to the pan-Canadian Patient Summary Interoperability Specification that presents the broader context for clinical, business, interoperability and solution development considerations that were discovered during the development of the PS-CA. It defines the healthcare problem that the PS-CA addresses and includes healthcare use cases and interoperability requirements in terms that will be traceable to the content in the pan-Canadian Patient Summary - Companion Guide to Reference Architecture, which defines the actors and their interactions with other actors and the pan-Canadian Patient Summary – FHIR Implementation Guide, which defines the contents and semantic interoperability of the PS-CA.
This document will also support upcoming releases and roadmap elements of the PS-CA specification.
Target Audience: CTOs, CMIOs, CIOs, PTs and vendors
- pan-Canadian Patient Summary - Companion Guide to Reference Architecture
The pan-Canadian Patient Summary - Companion Guide to Reference Architecture contains background information on the abstracted PS-CA actors and transactions for the Pan-Canadian Patient Summary Interoperable Specifications for stakeholders who are not familiar with the IHE Methodology. It describes baseline information on the recommended IHE profiles and includes links to the IHE source documentation where stakeholders can get additional details on each PS-CA actor and transaction. This document also includes descriptions of alternatives and choices for implementation patterns and ecosystem architectures to support the Patient Summary-CA in current state, including sequence diagrams that demonstrate the relationship and dependencies between the PS-CA actors and transactions.
Target Audience: CTOs, CMIOs, CIOs, PTs and vendor
Additionally, the following document also contains relevant background information for the Pan Canadian Patient Summary Interoperability Specifications v1 Trial Implementation
- Proposed pan-Canadian Interoperability Reference Architecture v1:
The Proposed pan-Canadian Interoperable Reference Architecture is intended as a conversation starter on the broader interoperability landscape, not limited to patient summaries. Its purpose is to facilitate multi-stakeholder dialogue, collaboration and convergence towards common standards. It is a conceptual roadmap that provides a common vocabulary and a set of abstracted actors and transactions representing typical components in a digital health ecosystem (public and private sector solutions). It is composed of building blocks based on international standards.
Target Audience: CTOs, CMIOs, CIOs, Technical Leads, Decision makers, PTs, vendors
This document fits into an overall specification framework described below
Description
This Interoperability Specification describes the technical interface requirements for sharing Patient Summaries in the scheduled and unscheduled local context in Canada.
Documents Convention
The Pan Canadian Patient Summary Interoperability Specifications v1 Trial Implementation will be numbered according to this format:
- Name + Version + Stage, where name refers to the name of the document, version refers to the versioning history of the document and stage refers to its stage in implementation such as “Trial Implementation” or “TI”.
- Key documents will evolve during review cycles from version 0.x to v1.0
In order to implement a system that fully supports the three Patient Summary-CA Use Cases and the Pan Canadian Patient Summary Interoperability Specifications v1 Trial Implementation, the system shall be able to demonstrate that it conforms to every mandatory actors and transactions for which it is claiming conformance in order to secure share Patient Summaries during scheduled and unscheduled local care for Release 1.
Requirements Language
The following conventions are used to specify requirement levels for the business requirements of the Pan Canadian Patient Summary Interoperability Specifications v1 Trial Implementation:
- SHALL: Requirement is Mandatory for Interoperability
- MAY: Requirement is Optional for Interoperability or Solution Functionality.
- SHOULD: Requirement is Recommended for Solution Functionality
Additional information on the PS-CA business requirements can be found in the Patient Summary – Companion Guide to Use Cases and Definitions.
Methodology
The Pan Canadian Patient Summary Interoperability Specification v1 Trial Implementation document has been co-developed with feedback and input from various jurisdictions and vendors collected during several months through Coordinating Table Meetings, Executive Table Meetings, stakeholder workshops and 1-on-1 meetings.
Stakeholders included clinicians, technical SMEs, standards SMEs from participating jurisdictions (e.g., AB, ON, BC, SK, and NL) and vendors, software developers and more from participating jurisdictions (e.g., AB, ON, BC, SK, and NL). The development the Patient Summary-CA specification relies on the business requirements set by the in-scope Use Cases of the PS-CA project. These high-level requirements are not restated in this specification. Stakeholders should review the Companion Guide for this information.
Introduction to a Use-Case Driven Approach
The following use-case driven approach was utilized in the development of the Pan Canadian Patient Summary interoperability Specifications v1 Trial Implementation:
- Baseline: Develop foundational Use Cases, Use Case Scenarios and Business Requirements for pan-Canadian Patient Summaries based on information provided by jurisdictions
- Collaborate: Collaborate with jurisdictions, clinical SMEs, technical SMES, vendors, participating organizations to develop and refine detailed artefacts
- Review: Review and provide feedback into artefacts through engagement workshops and input gathering
- Publish: Publish artefacts for broader stakeholder consultation
- Recommend: Recommend draft artefacts for approval
- Iterate: Continue to refine as per testing and priorities
Release Cycle
The pan-Canadian Patient Summary Specifications release cycle will include a multi-stage review and feedback process. For more information, please visit the pan-Canadian Interoperability PS-CA Release Information page.