In support of the provinces and territories, Canada Health Infoway (Infoway) is facilitating a national collaborative effort to advance interoperability. While there are many interoperability-related challenges, these specifications address the challenge of sharing Patient Summaries.
A Patient Summary (PS) is a health record extract comprising a standardized collection of clinical and contextual information (retrospective, concurrent, prospective) that provides a snapshot in time of a subject of care’s health information and health care, while a longitudinal health record is a single comprehensive patient record comprised of data from numerous data sources across the health care continuum.
Canada is not alone in trying to solve for this challenge. The International Patient Summary (IPS) project started in Europe several years ago and has been adopted by ISO, IHE and HL7 International. In addition, there is an active working group led by the Office of the National Coordinator (ONC) in the United States called the Global Digital Health Partnership (GDHP) actively working with its members on solutions for Patient Summary exchanges at an international scale. Canada is an active participant in this partnership. At the G7 meeting in June 2021, Canada committed to work with jurisdictions, vendors, and participating organizations to collaborate on a pan-Canadian implementable specification that aligns to the IPS and reflects Canada's jurisdictional realities. The overarching principle adopted for the Patient Summary-CA (PS-CA) project is to align as closely as possible to the IPS profiles. This principle will support jurisdictions in representing their clinical workflows and will support implementers in undergoing the necessary change management efforts required to support adoption activities, ensuring this work effort is a worthwhile investment.
In October 2021, the CHIEF Executive Forum, an influential Digital Health Canada member group, published a white paper, The Value of the International Patient Summary in Canada, providing an overview of IPS implementation globally, including lessons learned from other countries. Benefits and value to patients, clinicians, and the healthcare system are explored in the white paper and these lessons are translated into key themes for IPS in Canada, as well as a roadmap to successful implementation.
High-Level PS-CA Release Roadmap
The PS-CA Release Roadmap, as outlined in the table below, provides the current focus for the PS-CA, starting with supports for sharing Patient Summaries for local care with information from a single source. As the PS-CA journey unfolds, additional content will be added throughout the life of the PS-CA Roadmap to accommodate the requirements of Canada's implementers, such as the ability to consolidate Patient Summary information from multiple sources to create a single Patient Summary that can be exchanged internationally with target nations.
| Jurisdiction | Expected User Scenarios | Expected Implementation | High-Level Roadmap |
|---|---|---|---|
| Local |
|
| Current Focus (AB, BC, MB, NL, ON, SK) |
| Provincial |
|
| Future Focus (Subject to Change) |
| X-Provincial |
|
| |
| International |
|
|
PS-CA for Trial Implementation
The first PS-CA Trial Implementation release will include supports for sharing Patient Summaries for scheduled or unscheduled local care with information from a single source.
The PS-CA specifications implementation approach for alignment with the IPS will span a number of releases on a shared pan-Canadian interoperability roadmap. The first Trial Implementation release will focus on three use cases that have been identified as priority by the participating Canadian jurisdictions (i.e., Alberta (AB), British Columbia (BC), Ontario (ON), Saskatchewan (SK) and Newfoundland & Labrador (NL)) and their supporting business requirements, actors and transactions, terminology and FHIR® profiles. The following figure represents the:
- alignment of the PS-CA to the IPS-UV,
- data domains of interest by the participating Canadian jurisdictions (including Manitoba (MB), according to their Home Clinic Client Summary Service); and
- data domains included in the PS-CA specifications and data domains planned for future releases.
Legend
- Blue: Header domains
- Red: Required domains
- Orange: Recommended domains
- Green: Optional domains
- Grey: Domains of interest by jurisdiction
- White: Domains not identified by jurisdictions as priority and/or not included in the PS-CA v1.0.0 TI release
Context
The PS-CA specifications are published to a public space within Canada Health Infoway’s InfoScribe and are also available in downloadable PDF format. InfoScribe is a web-based tool developed for jurisdictions and vendors to create, publish, and collaborate on clinical requirements and specifications for interoperability solutions. Teams can document, share, and discuss content, files, ideas, specs, mock-ups, diagrams, and projects. A link to the online published content and the downloadable documentation will be published with each release of the PS-CA.
Release information for each release is contained in the corresponding PS-CA Release page.
New content will be added throughout the life of the PS-CA Roadmap to accommodate the requirements of Canada's implementers.
Introduction to IHE
Integrating the Healthcare Enterprise (IHE) is an international initiative to promote the use of standards to achieve interoperability among health information technology (HIT) systems, and the effective use of electronic health records (EHRs). IHE provides a forum for care providers, HIT experts and other stakeholders in several clinical and operational domains to reach consensus on standards-based solutions to critical interoperability issues.
The primary output of IHE are system implementation guides, called IHE profiles. IHE publishes each profile through a well-defined process of public review and Trial Implementation, and gathers profiles that have reached Final Text status into an IHE Technical Framework. These profiles are referenced in the Appendices of this document.
Preference for Modern HL7 FHIR interfaces
New implementation of IHE profiles based on the PS-CA should avoid legacy interfaces. IHE profiles that are HL7 FHIR based are preferred when available; however, the Reference Architecture will account for legacy systems that do not support FHIR. Canada Health Infoway will encourage the adoption of modern exchange protocols but will also provide the runway and opportunity for the jurisdictions to improve their interoperability capabilities.
For implementation patterns pertaining to HL7 FHIR Health Information Exchange (HIE) patterns, a new pan-Canadian Interoperability Specifications document has been developed (i.e., CA:FeX). More information about CA:FeX can be found in the Companion Guide: Reference Architecture of the PS-CA specifications and also in the pan-Canadian Interoperability CA:FeX pages here.
How to Read This Document
This document contains the following content, as well as informative appendices for your convenience.
- Preface: Contains an introduction to the pan-Canadian Patient Summary Interoperability Specifications. This section contains a summary of the context, document purpose and scope, as well as other content to help orient the first-time reader to the topic of these specifications and how they relate to other specifications in the digital health ecosystem in Canada.
- PS-CA Use Case Overview: Describes the Use Cases, including design constraints and assumptions and the flows of information that will be specified in the PS-CA specifications. This section also introduces scenarios that describe how the specified flows may be used in the Canadian context.
- Core Interoperability Specifications: Establishes the Core Interoperability Requirements for the PS-CA for two implementation options:
- Document Repository/Registry Pattern (i.e., MHD - IHE Profile); and
- FHIR Health Information Exchange (HIE) Pattern (i.e., CA:FeX).
- PS-CA Actor Conformance: Establishes the Conformance Requirements for the PS-CA specifications for the two implementation patterns identified above.
- Privacy & Security Guidance: Provides a reference to Infoway's recently published privacy primer, Privacy as an Enabler, that provides an introduction to interoperability, an overview of Canadian privacy laws and some practical approaches to privacy for interoperability. And, it provides a high-level list of security considerations for the PS-CA specifications.
- Information Models, Applications and Infrastructure: Provides key implementation guidance around Information Models, Applications and Infrastructure for the PS-CA specifications.
- PS-CA Content Data Model & FHIR® Profiles: Describes the required PS-CA Content Data Model & FHIR® Profiles.
Related Documents & References
The pan-Canadian Patient Summary Interoperability Bundle is the sole entry point for the technology developers, the compliance assessment testing and certification, and the purchasers of IT systems in terms of technical requirements.
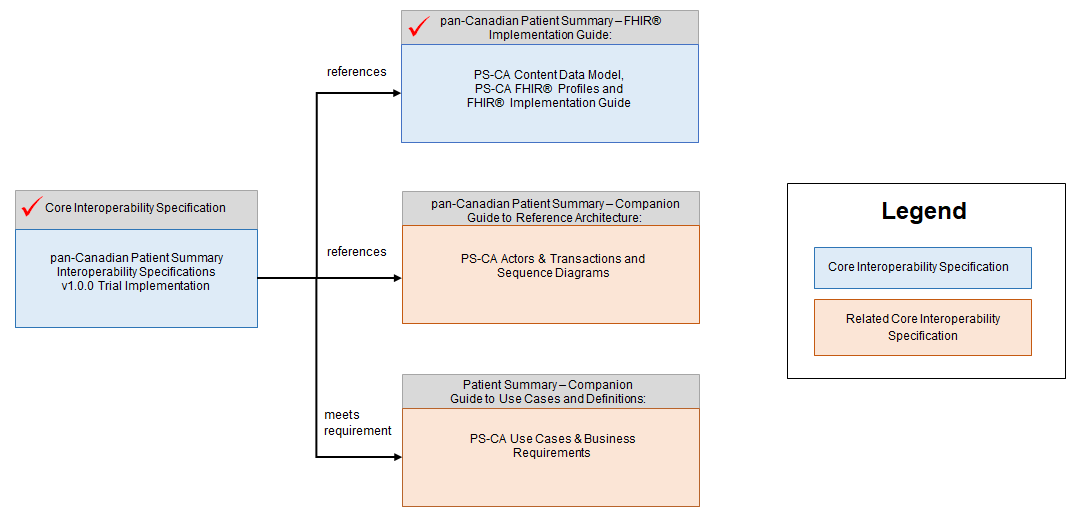
The PS-CA specifications reference several supporting documents:
- pan-Canadian Patient Summary – FHIR Implementation Guide
The pan-Canadian Patient Summary - FHIR Implementation Guide is an implementable, testable specification for the HL7 FHIR composition that defines the data payload of the PS-CA specifications, based on the HL7® FHIR® IPS implementation guide. It contains information for solution developers to implement the PS-CA content data model using the HL7® Fast Healthcare Interoperability Resources (FHIR®) standard. It describes the data elements & types, cardinality, constraints, and code system references - all of the details needed for two systems to be semantically interoperable with each other when a PS-CA compliant patient summary is exchanged.
Target Audience: Solution Developers
- pan-Canadian Patient Summary - Companion Guide to Use Cases & Definitions
The pan-Canadian Patient Summary - Companion Guide to Use Cases & Definitions is a companion document to the pan-Canadian Patient Summary Interoperability Specifications that presents the broader context for clinical, business, interoperability and solution development considerations that were discovered during the development of the PS-CA. It defines the healthcare problem that the PS-CA addresses and includes healthcare use cases and interoperability requirements in terms that will be traceable to the content in the pan-Canadian Patient Summary - Companion Guide to Reference Architecture, which defines the actors and their interactions with other actors and the pan-Canadian Patient Summary – FHIR Implementation Guide, which defines the contents and semantic interoperability of the PS-CA.
This document will also support upcoming releases and roadmap elements of the PS-CA specifications.
Target Audience: CTOs, CMIOs, CIOs, PTs, Health Care Providers and vendors
- pan-Canadian Patient Summary - Companion Guide to Reference Architecture
The pan-Canadian Patient Summary - Companion Guide to Reference Architecture contains background information on the abstracted PS-CA actors and transactions for the pan-Canadian Patient Summary Interoperability Specifications for stakeholders who are not familiar with the IHE Methodology. It describes baseline information on the recommended IHE profiles & pan-Canadian Interoperability Specifications and includes links to the Reference Architecture, available here: RA v0.1.0 DFT, where stakeholders can get additional details on each PS-CA actor and transaction. This document also includes descriptions of alternatives and choices for implementation patterns and ecosystem architectures to support the Patient Summary-CA in current state, including sequence diagrams that demonstrate the relationship and dependencies between the PS-CA actors and transactions.
Target Audience: CTOs, CMIOs, CIOs, PTs and vendors
PS-CA Specifications Package
Document Conventions
The pan-Canadian Patient Summary Interoperability Specifications will be versioned according to the IO Specifications Publication Model, defined here.
Requirements Language
The following conventions are used to specify requirement levels for the business requirements within the specifications:
- Shall: used to indicate a required requirement.
- Should: used to indicate that a requirement is recommended and should be considered as best practice for implementation, but not required (i.e., it is optional) for implementation.
- May: used to indicate that a requirement is permittable / optional, but not required for implementation.
- Shall not: used to indicate that an element or action is prohibited.
Additional information on the PS-CA business requirements can be found in the Patient Summary – Companion Guide to Use Cases and Definitions.
Methodology
The Specifications have been co-developed with feedback and input from various jurisdictions and vendors collected during several months through Coordinating Table Meetings, Executive Table Meetings, stakeholder workshops and 1-on-1 meetings.
Stakeholders included clinicians, technical SMEs, and standards SMEs from participating jurisdictions (i.e., AB, ON, BC, SK, and NL), as well as vendors and software developers. The development of the PS-CA specifications rely on the business requirements set by the in-scope Use Cases of the PS-CA project. These high-level requirements are not restated in the specifications. Stakeholders should review the Companion Guide: Use Cases and Definitions for this information.
Introduction to a Use-Case Driven Approach
The following use case-driven approach was utilized in the development of the pan-Canadian Patient Summary Interoperability Specifications:
- Baseline: Develop foundational Use Cases, Use Case Scenarios and Business Requirements for pan-Canadian Patient Summaries based on information provided by jurisdictions
- Collaborate: Collaborate with jurisdictions, clinical SMEs, technical SMES, vendors, participating organizations to develop and refine detailed artefacts
- Review: Review and provide feedback into artefacts through engagement workshops and input gathering
- Publish: Publish artefacts for broader stakeholder consultation
- Recommend: Recommend draft artefacts for approval
- Iterate: Continue to refine as per testing and priorities
Release Cycle
The PS-CA specifications' release cycle includes a multi-stage review and feedback process. For more information, please visit the pan-Canadian Interoperability PS-CA Release Information page.