LOINC ® Registered Trademarks
LOINC is a registered trademark of the Regenstrief Institute Inc.
LOINC and RELMA are registered United States trademarks of Regenstrief Institute, Inc.
Full LOINC and RELMA Terms of Use are available at: https://loinc.org/kb/license/
What is LOINC?
- LOINC is a terminology that is used to standardize lab tests and other observations
- Each LOINC record corresponds to a single test result, panel or document
- Records identify observations such as:
- laboratory test results
- other diagnostic service test results
- clinical observations and measurements
- reports produced by clinicians
- diagnostic services about patients
- panels, forms and collections that define aggregations of these observations
- orders for these entities in electronic reports and messages
- Used ‘beneath the covers’ in electronic systems to standardize observables in lab and other clinical domains
Why is LOINC Important?
- LOINC provides a set of universal names and identification codes for standardizing laboratory and clinical observables
- Most systems have idiosyncratic names, receiving medical systems cannot fully “understand” the information they receive from multiple systems unless they adopt each sender’s terminology or invest in the work to map each systems terms to their internal code system
- This issue could be resolved if those who need to communicate adopt LOINC codes to identify their observables
CDA LOINC Codes
- The HL7 CDA is a structured document specification
- It specifies the structure and semantics of clinical documents for the purpose of exchange
- A CDA document can contain any type of clinical content
- Discharge Summary, Imaging Report, Admission & Physical, Pathology Report, etc
- LOINC is used anywhere in a CDA document when a document type needs to be identified
- Included in the header and the body of a CDA document
- LOINC document type Codes provide consistent semantics for names of documents exchanged between systems
- What is a document?
- A collection of information
- Contains a title, sections, sentences and other content
- It is not a Panel
- Panels contain enumerated, discrete element
- Kinds of Documents:
- Clinical Note
- A clinical document, produced by clinicians spontaneously or in response to a request for consultation.
- Clinical Report
- A clinical document, produced in response to an order for a procedure
- Formal Document Ontology exists in LOINC for Clinical Notes today
LOINC Content - General
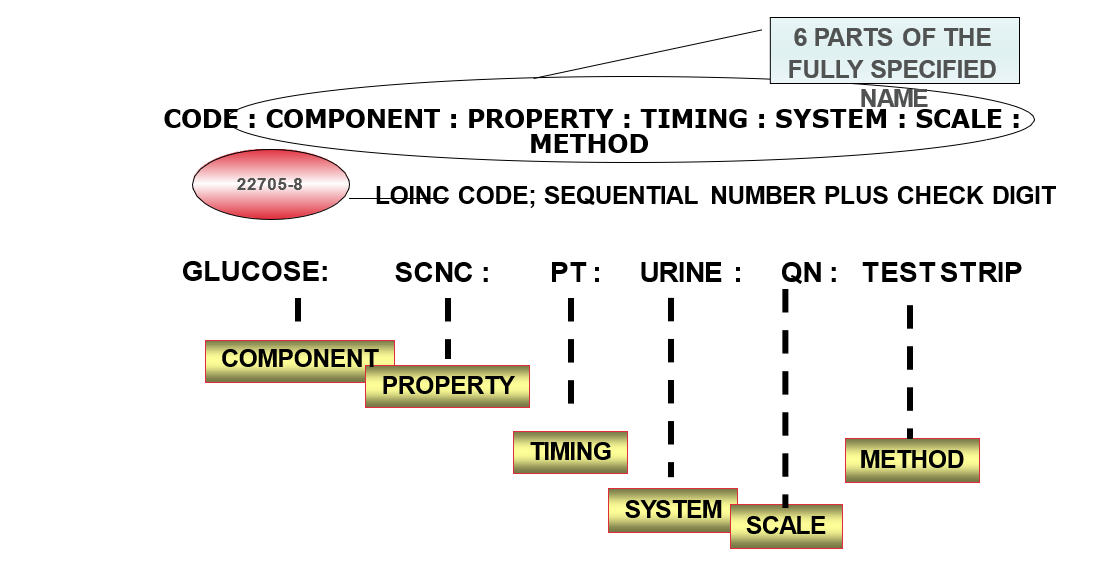
Each record contains:
- 6 part formal name, plus LOINC numerical number with check digit
- More than 50 columns to either further distinguish the record or provide information about the record to help implementers. Examples include:
- Related Names
- Example Units
- Class –CHEM, HEM/BC, OBGYN, PATH etc.
- LOINC Long Common name
- LOINC Short name
- Records are never deleted
What is NOT part of a LOINC Record
- The instrument used in testing
- Specific details about the specimen (right arm etc)
- Priority (such as STAT)
- Where the testing is done
- Who did the test
- Anything that is not an intrinsic part of the observation
- Other data is communicated in specific places of the electronic message
Core elements of a LOINC Record