Release Scope
- Request Requests for change received change received from Canadian implementers from September 152, 2018 2023 to March 14February 2, 2019 2024 - InfoRMS SNOMED CT RFC Project
- Requests to add, change or inactivate content in both English and French French.
- Content corrections Corrections to content (typographical errors, metadata, others) .
- CA EN CA concepts promoted to international version the International Edition of SNOMED CT.
- In the Release Notes, "Issues in progress" refers to requests that were accepted and added to the most recent Canadian Edition, but they also have been submitted to SNOMED International for consideration for core inclusion. If accepted, they are in scope for inclusion in the International Edition and will be assigned to a release by SNOMED International and will require harmonization with the next CA Edition release.
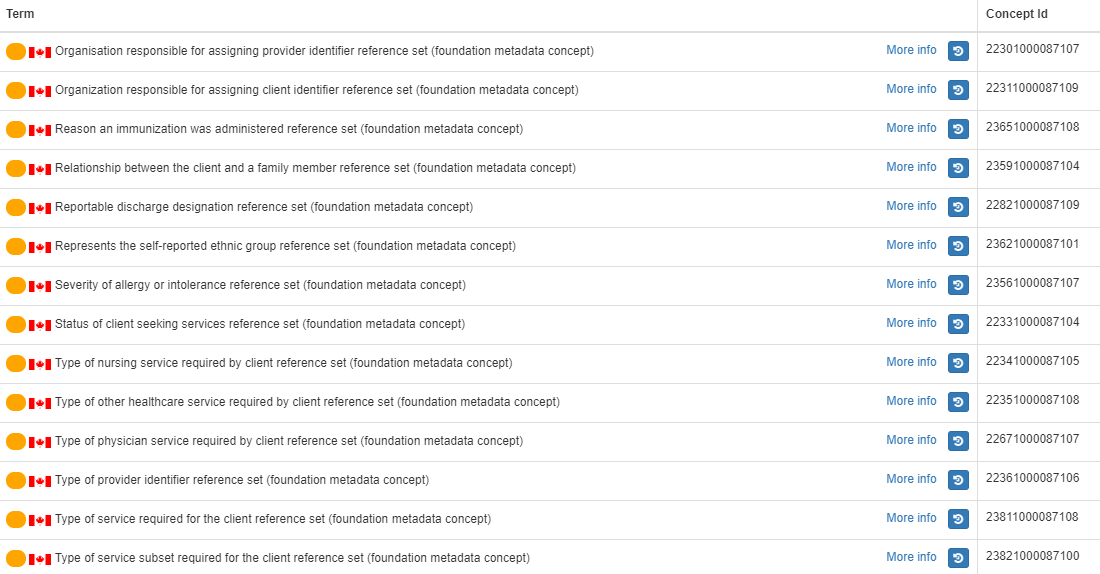
- Subsets - Subsets - InfoRMS Subset RFC Project
- Pan-Canadian SNOMED CT subsets in scope for this maintenance cycle include: Immunization, Communicable Disease
- Subsets updated: Immunization subsets, Primary Health Care subsets and the Communicable Disease subsetothers.
- New subset created: PrescriptionMedicationCoverage and PrescriptionIndicationForUse
- No subsets inactivated
- 29 SNOMED CT subsets (refsets) are published as extensional refsets in the Canadian Edition (Canada Health Infoway Simple Type Reference Set (foundation metadata concept)).
- 23 of 52 subsets are expanded type subsets that are intensionally defined (i.e. defined using queries). These can be found as members of The CA Edition Refset module contains the filter definition for 14 of the extensional published reference sets. They can be found in the Simple query specification reference set (foundation metadata concept). Note that all reference sets are also published as a simple reference set (enumerated).
The Canadian Edition of SNOMED CT was "modularized" to allow
- Selection of the terminology "products" according to the different audiences requirements. For example: not all stakeholders require the French content. Publication of different Canadian terminology content in different release cycles. This pattern enables the tracking of dependencies between releases from different modules that are related but asynchronous. For example: Update and publish reference sets (subsets) that are impacted by terminology changes or when new CA Edition content has been developed.
- The Canadian English extension, the basic component
- The Canadian French extension, as an optional component The Canadian extension Refsets. They were distributed in a separate module since they could require more frequent release cycles than the basic component.
- This change will simplify the modeling of Canadian content This change will allow for a more agile release cycle
- Promote the refsets to the basic Canadian English module. The Canadian Edition will then be composed of two modules, instead of three.
- The identifiers of the refsets and the refset members would not change, they would be moved to the basic en-CA module.
- The change is not expected to significantly impact implementations as the component identifiers would be moved to a higher level extension and transparently available for routine usage.
- OWL DL Enhancement To enable improvements to the quality and analytics capabilities of SNOMED CT, SNOMED International is enhancing the underlining logic profile of the terminology. The changes require expressing the concept definitions using Web Ontology Language (OWL). These expressions will be published as an RF2 compliant reference set, replacing the stated relationships file. It is planned that the stated relationships file will be deprecated in the July 2019 International release.
- As of the July 2019 release of SNOMED CT, the stated relationships file will be inactivated and replaced by one OWL expression refset. The profile for the extended set of features SNOMED CT will use is defined at http://snomed.org/lps. While the stated relationship file is still present and individual concept definitions do not yet use extended features, the content of the inferred relationships file will be affected in the January 2019 release. Any implementations performing subsumption testing based on the distributed inferred relationship file may be affected and should be reviewed and carefully tested prior to upgrading. Proposed change and guidance:
- There is a refset OWL expression file added in each of the release types of the CA Edition.
- If you need to classify, please contact Infoway for support and guidance.
Since 2016, the SNOMED CT Canadian Edition RF2 baseline content has been distributed in three modules:
In 2019, simplification of the module dependencies is desirable to simplify the development, management, publication and implementation of the Canadian Terminology content.
Proposed change:
You may be affected by the coming changes if your implementation requires classification or rely on relationships and subsumptions. In fact, the changes being introduced expand the Description Logic features used to define concepts in SNOMED CT. These changes affect the RF2 distribution format, and particularly the stated and inferred relationships file content and interpretation.
- As part of the PrescribeIT Project many subsets have been updated with the translation of French synonyms. These French terms were added to different code systems such as UCUM, HL7 FHIR and SNOMED CT.
- These synonyms have been added to PrescribeIT subsets V.3 (SR-145)
- As part of the PrescribeIT Project many subsets have been updated with the translation of French synonyms. These French terms were added to different code systems such as UCUM, HL7 FHIR and SNOMED CT.
- Validation of existing translated synonyms has been completed by Dr Beauchemin
- On behalf of Infoway, a warm thank you Dr Beauchemin for providing clinical expertise and guidance for this review.
- This review was done in order to assure high quality of translation where Canadian translation differed from the SNOMED International French Starter Set and/or the France PHAST translation
- All issues were reviewed and outcomes documented in the Informs RFC tickets: BSCT-8534, BSCT-7338, BSCT-8098.
- Validation of existing translated synonyms has been completed by Dr Beauchemin
- The French Editorial Guidelines have been updated.
- See below printscreen.
Notes for this Release:
- New vaccine concepts to support the ongoing work for Immunization.
- Release notes for International releases including the International Edition and the Common French extension are no longer included in the Canadian Edition release package. International release notes can be accessed here and Common French release notes can be accessed here.
- Inclusion of concepts that originated in other national extensions and were promoted to core because they also meet Canadian requirements and, as such, meet the international use case (lateral promotion).
- Two (2) new subsets were created to support the new National Vaccine Catalogue (NVC).
- In line with the new implementation of Annotations, two new refsets have been added to the International Edition Release package, from December 2023 onwards:
der2_scsRefset_ComponentAnnotationStringValueSnapshot_INT_20240101.txt
der2_sscsRefset_MemberAnnotationStringValueSnapshot_INT_20240101.txt
These refset files are empty for the February 2024 release, whilst content is authored in future editing cycles. However, they will then be populated with Annotations data from one of the future 2024 International Edition releases onwards (date to be confirmed). These improvements were introduced in the December 2023 International Edition release, and will be used in all future International Edition releases until further notice. - French translation to support Canadian implementations, including: ongoing immunization work; the Canadian Institute for Health Information (CIHI) pan-Canadian Health Data Content Framework (pCHDCF) and Primary Health Care projects; Ontario Health’s Lab Information System (OLIS) and Provincial Health Service Directory (PHSD); other projects from Ministère de la Santé et des Services sociaux du Québec (MSSS) such as CNESST, CISSSMO, PTI and Synoptic colonoscopy report.
Refer to section 5. Questions and Comments, for additional information
Known issuesThe recent Immunization request for change (to change the Manufacturer for MAH) has targeted only a small number of concepts. Infoway is aware that other concepts should be modified. The review of potential changes will be done for the next CA Edition release.
Refer to section Questions and Comments, if you want yo know more about SNOMED CT or if you have comments for us.
Refer to section 6. Requests for Change (RFC), if you need more for additional information on how to request on terminology content or subsets.
Refer to section section 7. Updates and Publications, if you want to be aware of when changes are published for dates and times related to publication.